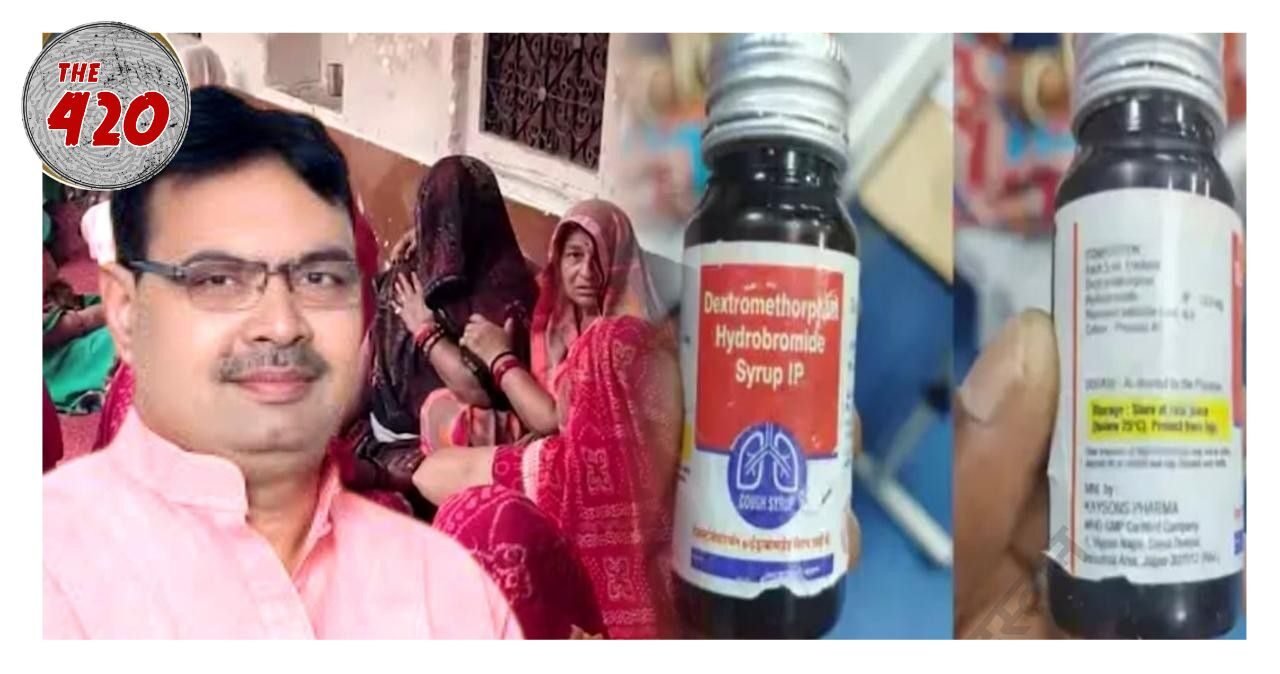
A wave of anger and grief has gripped parts of Rajasthan after several children reportedly died following the consumption of a contaminated cough syrup, with families now alleging that officials attempted to tamper with evidence to cover up the incident.
According to local media reports, the deaths occurred shortly after the children were administered a cough syrup labeled Dextromethorphan Hydrobromide Syrup IP, allegedly manufactured by a Haryana-based pharmaceutical company.
FCRF Launches CCLP Program to Train India’s Next Generation of Cyber Law Practitioners
The families claim that health department officials visited their homes soon after the incident and removed the labels from the remaining syrup bottles an act they believe was intended to destroy critical evidence linking the deaths to the product.
Families Accuse Officials of Cover-Up
Relatives of the victims told reporters that instead of collecting samples for investigation, the visiting officers allegedly took away the bottles and stripped their labels, preventing proper identification of the product and its batch number.
“They came saying they wanted to check the medicine, but they took away the bottles and tore off the labels,” one grieving parent alleged. “If they were investigating, why hide the name of the company?”
The families have demanded a judicial inquiry into both the cause of death and the alleged misconduct by officials. They insist that only an independent probe can bring out the truth about the potential link between the cough syrup and the fatalities.
Health Department Yet to Issue Clarification
As of now, the Rajasthan Health Department has not released any official statement on the incident. Sources said that internal teams have been instructed to verify the manufacturing and distribution chain of the cough syrup, particularly focusing on whether it was locally procured or part of a larger supply batch distributed across other states.
Pharmaceutical safety experts say that if the allegations are proven true, it could amount to a criminal violation under the Drugs and Cosmetics Act, carrying severe penalties for both the manufacturer and local enforcement officials.
The case also raises broader concerns about drug quality monitoring and post-market surveillance in rural India, where medical products often escape stringent testing and inspection.
Echoes of Past Controversies
This incident draws uncomfortable parallels with similar tragedies in India’s past including the 2019 Udhampur case and the 2022 Uzbekistan tragedy, where children died after consuming adulterated syrups containing toxic compounds like diethylene glycol.
Experts have long warned that unregulated small-scale pharmaceutical manufacturing and weak enforcement of quality control create loopholes that allow unsafe drugs to reach vulnerable populations, particularly in rural areas.
“If these deaths are linked to toxic contamination or poor formulation, it would represent yet another preventable failure in the country’s drug safety ecosystem,” said a senior pharmacologist familiar with regulatory audits in North India.
Call for Transparency and Accountability
Public outrage continues to mount on social media, with citizens demanding immediate arrests and an independent forensic examination of the syrup. Civil society groups have also urged the government to make all test results public and take steps to strengthen drug regulation mechanisms, including surprise inspections and real-time tracking of pharmaceutical batches.
As grieving families await answers, the Rajasthan cough syrup tragedy underscores a recurring pattern of systemic negligence, delayed response, and alleged cover-ups that continue to erode public trust in India’s health governance framework.